Intraoperative Neurophysiological Monitoring in Corrective Surgery of Scoliosis – Experience at a Tertiary Care Hospital
By Sajid Ali, Muhammad Talha, Muhammad Asad Qureshi, Waseem Afzal, Babar Shamim, Shahzad InamAffiliations
doi: 10.29271/jcpsp.2024.03.284ABSTRACT
Objective: To evaluate the role of intraoperative neurophysiological monitoring (IONM) in reducing the postoperative neurologic deficit following corrective surgery of scoliosis.
Study Design: Observational Study.
Place and Duration of the Study: Spine Surgery Department, Combined Military Hospital, Rawalpindi, from December 2022 to May 2023.
Methodology: The study included 170 cases of scoliosis operated under multimodal IONM. Decreased amplitude of ≥50% in SSEP or 70-80% in MEPs were considered warning signs. Cases were divided into two groups: Group 1 (signal drop) and Group 2 (no signal drop). Group 1 was subdivided into Group 1a (true positive), Group 1b (false positive) and Group 1c (intermediate positive). Group 2 was subdivided into Group 2a (true negative) and Group 2b (false negative).
Results: Evoked potential changes were observed in 27 (15.9%) cases. This includes transient drop of signals in 16 (9.4%) and sustained drop of signals in 11 (6.5%) cases. Among sustained signal drop, 9 (5.29%) cases had exhibited postoperative neurological deficit whereas 2 (1.17%) cases did not show postoperative neurological deficit (false positive). Multimodal IONM in the current study shows sensitivity of 100%, specificity of 98.6%, positive predictive value of 92.6%, and negative predictive value of 100%.
Conclusion: Multimodal IONM reduces the incidence of postoperative neurological deficit in corrective surgery of scoliosis by effectively detecting neurologic injury during surgery. Monitoring events alert surgical team to exercise immediate corrective measures which likely results in recovery of lost signals and predict the favorable outcome.
Key Words: Intraoperative monitoring, Motor evoked potentials, Neurological deficit, Scoliosis, Somatosensory evoked potentials.
INTRODUCTION
Surgical correction of scoliosis is a complex spinal surgery that involves extensive manipulation of the vertebral column and accompanying neural structures. The procedure carries a great risk of complications among which the most feared one is postoperative neurological (sensory/motor) deficit.1 Literature reports about 23% patients with adult spinal deformity surgeries experience some kind of neurological deficit postoperatively.2 Risk factors include excessive spinal cord stretching, compression or stretching of the nerve roots, ischaemia of the cord and implant-induced direct injury to the spinal cord or nerve roots.3 The permanent neurological deficit can be avoided by early detection of damage at a reversible stage allowing surgeon to take prompt corrective measures.4
In order to minimise postoperative neurological deficit, intraoperative neurophysiologic monitoring (IONM) was introduced in late 1970s and has become the standard practice in complex spinal surgeries, especially when instrumentation and deformity correction are to be done.2
Intraoperative neurophysiological monitoring apprises real time neurologic status of the central nervous system, nerve roots and peripheral nerves of anaesthetised patients.5 It rapidly identifies neural injury and alert surgical team to intervene and reverse an otherwise permanent deficit.6 Currently most frequently used IONM modalities includes transcranial motor evoked potentials (TcMEPs) which assess integrity of motor component of nervous system,7 somatosensory evoked potentials (SSEPs) which assess integrity of sensory component of nervous system from peripheral nerves to somatosensory cortex,8 spontaneous electromyography (s-EMG) and triggered electromyography (t-EMG) monitor individual nerve root trauma and pedicle breach during placement of screws,9 brainstem auditory evoked potentials (BAEPs) and visual evoked potentials (VEPs). The modalities mentioned can be used individually however, the multimodality approach is widely accepted for broader picture of various components of nervous system monitoring.2 In 2009, Quraishi et al. conducted prospective study on 102 cases of adult spinal deformity surgery under multimodality IONM (MIONM) technique. They have reported an overall sensitivity of MIONM of 100% and specificity of 84%.10
The present study was designed to ascertain the usefulness of IONM in early detection of signal loss during corrective surgery of scoliosis and to assess the improvement of neurological signals after prompt corrective interventions.
METHODOLOGY
This observational study was conducted at the Department of Spine Surgery, Combined Military Hospital, Rawalpindi, from December 2022 to May 2023. Formal permission was obtained from the Ethical Review Committee of the Combined Military Hospital, Rawalpindi (IRB Serial No. 391 dated 2 December 2022) prior to initiation of the research. A total number of 170 cases of corrective surgery of scoliosis operated under IONM from February 2015 to December 2021 were included in the study. After getting informed verbal consent from the patients on phone calls to participate in the study, data of patients and IONM findings were retrieved from database of the department and internal memory of neurophysiologic workstation (Xltek protector 32 IOM system), respectively.
Inclusion criteria include age ranges between 4 to 21 years, either gender, scoliosis curve in the range of 60 - 120 degrees. The patients with neurologic deficits prior to the surgery or any neuromuscular junctional disease affecting impulse transmission were excluded from the study. Multimodality approach (including SSEP, TcMEP, and s-EMG) was adopted for monitoring of cases. All the surgeries were performed under total intravenous anaesthesia (TIVA) which was maintained with propofol infusion at 100 μg/kg/min and infusion of dexmedetomidine hydrochloride 0.8 μg/kg/hr. Bispectral index (BIS) was kept in the range of 30-45 with dose adjustments of these anaesthetic agents. In order to facilitate intubation, muscle relaxant atracurium (0.5 mg/kg) was used once only at the time of induction. Effect of muscle relaxants on neurophysiological response was ruled out by conducting train of four (TOF). Baseline recordings of SSEPs and MEPs were obtained before giving incision to the patient.
All the surgeries were monitored with neurophysiologic workstation (Xltek protector 32 IOM system; Natus Medical Inc., Oakville, Canada). The cortical SSEPs were elicited bilaterally by stimulation of posterior tibial and median nerves through surface electrodes using a 500 microsecond square wave electrical pulse at a rate of 3.9 hertz (Hz). The intensity of stimulus was kept in the range of 20 - 35 mA and bypass filter set at 30 to 500 Hz. Recording of cortical potentials was obtained through sub-dermal corkscrew electrodes applied on the scalp at points Fpz, Cz, C3, and C4 according to the international 10 - 20 system. Either ≥50% decrease in the amplitude or ≥10% increase in the latency of signals were considered alarm sign. Bilateral TcMEPs were recorded from the targeted muscles of upper and lower limbs as per individual requirements. Frequently monitored muscles of the upper limbs include brachioradialis and abductor pollicis brevis and of the lower limb include illiopsoas, adductor magnus, quadriceps femoris, tibialis anterior, extensor hallucis longus and abductor hallucis. The response was triggered through sub-dermal corkscrew electrodes applied on the scalp corresponding to the motor cortex at points C1-C2 and C3-C4 stimulating montage according to the international 10 - 20 system with stimulus intensity ranging 250 to 400 volts and anodal pulse train between 6 to 9 at an interval of 1 to 10 milliseconds. Responses were recorded from needle electrodes applied at targeted muscles. A complete loss of signals or decrease in signal amplitude by 70 to 80% was considered neurophysiological warning. Spontaneous EMG activities were continuously monitored throughout the surgery in both upper and lower limbs. Observation of sustained high- frequency neurotonic EMG activities leads to the initiations of formal warnings to the surgical team.
Based on IONM interpretations, cases were divided into two main groups, Group 1 (signal drop; n=27) and Group 2 (no signal drop; n=143). Group 1 is subdivided into Group 1a (true positive; n=9) in which sustained signal drop was observed which persisted even after exercising all the corrective measures and resulted in new postoperative neurological deficit, Group 1b (false positive; n=2) in which sustained signal drop was observed which persisted even after exercising all the corrective measures but no new postoperative neurological deficit was observed in the patient and Group 1c (intermediate positive/rescued cases; n=16) in which transient signal drop was observed which was restored by prompt corrective measures without any new postoperative neurological deficit. Similarly, Group 2 is subdivided into Group 2a (true negative; n=143) in which neither signal drop nor postoperative neurological deficit was observed and Group 2b (false negative; n=0) in which no signal drop was observed during the entire course of surgery but the patient developed new postoperative neurological deficit.
The data were analysed using IBM Statistical Package for the social sciences (SPSS) version 23.00. Numerical variables were reported as Mean ± SD whereas categorical variables were presented as numbers and percentages. To determine significance of sustained signal drop, Fisher’s exact test and Chi-square test were applied. A p-value of <0.05 was considered statistically significant.
RESULTS
The study was conducted on 170 cases out of which 71 (41.8%) were males and 99 (58.2%) were females. The age of patients ranges between 4–21(14.6±3.76) years. Out of the 170 studied cases, 79 (46.5%) were diagnosed cases of congenital scoliosis, 83 (48.8%) idiopathic scoliosis, and 8 (4.7%) cases were of neuromuscular scoliosis. As per the warning criteria mentioned earlier, changes in evoked potentials (signal drop) were observed during the surgery in 27 (15.9%) cases. These changes were either transient drop of signals in 16 (9.4%) cases or sustained drop of signals in 11 (6.5%) cases (Figure 1, Table I).
Table I: Frequency of evoked potential changes among different groups of scoliosis.
|
Groups |
Type of Scoliosis |
Total |
|||
|
Congenital |
Idiopathic |
Neuromuscular |
|||
|
Group 1 |
1a |
8 (10.1 %) |
1 (1.2 %) |
0 |
9 (5.3%) |
|
1b |
2 (2.5%) |
0 |
0 |
2 (1.2%) |
|
|
1c |
9 (11.4%) |
5 (6%) |
2 (25%) |
16 (9.4%) |
|
|
Group 2 |
2a |
60 (75.9%) |
77 (92.8%) |
6 (75%) |
143 (84.1%) |
|
2b |
0 |
0 |
0 |
0 |
|
Table II: Summary of patients with evoked potential changes and neurological deficits.
|
Patient No. |
Age (years) |
Gender |
IONM Changes |
Recovery |
Post-op Deficit |
||
|
TcMEP |
SSEP |
s-EMG |
|||||
|
1. |
10 |
F |
Lt QF,TA, AH |
Lt |
N |
Complete |
N |
|
2. |
20 |
F |
Rt AM,QF,TA,AH |
Rt |
N |
Complete |
N |
|
3. |
9 |
F |
Both AM,QF,TA,AH |
Both |
N |
Complete |
N |
|
4. |
13 |
F |
Both AM,QF,TA,AH |
Both |
Rt AH |
Partial |
Bilateral LLW. Power 3/5 |
|
5. |
13 |
M |
Rt TA, AH |
N |
N |
Complete |
N |
|
6. |
10 |
F |
Lt QF,TA,AH |
Lt |
N |
N |
Lt LLW. Power 3/5 |
|
7. |
18 |
M |
Lt AM,QF,TA,AH |
Lt |
N |
Complete |
N |
|
8. |
5 |
F |
Lt QF,TA, EHL,AH |
N |
N |
Complete |
N |
|
9. |
12 |
M |
Lt QF,TA,AH |
N |
N |
Complete |
N |
|
10. |
11 |
F |
Both TA,AH |
N |
N |
Complete |
N |
|
11. |
5 |
F |
Both AM,QF,TA,AH |
N |
N |
Complete |
N |
|
12. |
6 |
F |
Rt QF,TA,AH |
Rt |
N |
Complete |
N |
|
13. |
4 |
M |
Rt AM,QF,TA,AH |
N |
N |
Complete |
N |
|
14. |
13 |
F |
Both AM,QF,TA,AH |
N |
N |
Complete |
N |
|
15. |
5 |
F |
Lt AM,QF,TA,EHL |
Lt |
N |
Complete |
N |
|
16. |
16 |
F |
Rt AM,QF,TA,AH |
N |
N |
Complete |
N |
|
17. |
13 |
F |
Both AM,QF,TA,AH |
N |
N |
Complete |
N |
|
18. |
21 |
M |
Lt QF* |
N |
N |
Partial |
N |
|
19. |
17 |
F |
Lt AH* |
N |
Lt AH |
Partial |
N |
|
20. |
9 |
M |
Both AM,QF,TA,AH |
Both |
N |
Partial |
Rt LLW. Power 3/5 |
|
21. |
13 |
F |
Both QF,TA,EHL,AH |
Both |
N |
N |
Bilateral LLW. Power 2/5 |
|
22. |
15 |
F |
Lt AM,QF,TA,AH |
Lt |
N |
Partial |
Lt LLW. Power 3/5 |
|
23. |
18 |
M |
Both AM,QF,TA,AH |
Both |
N |
N |
Bilateral LLW. Power 3/5 |
|
24. |
16 |
M |
Both AM,QF,TA,AH |
Both |
Lt QF |
Complete |
N |
|
25. |
14 |
F |
Both AM,QF,TA,AH |
Both |
N |
N |
Bilateral LLW. Power 2/5 |
|
26. |
16 |
M |
Both AM,QF,TA,AH |
LT |
N |
N |
Bilateral LLW. Power 3/5 |
|
27. |
18 |
M |
Rt QF,TA, EHL, AH |
N |
N |
N |
Rt LLW. Power 3/5 |
|
* False positive case. AM, Abductor magnus; QF, Quadriceps femoris; TA, Tibialis anterior; EHL, Extensor hallucis longus; AH, Abductor hallucis; Lt, Left; Rt, Right; M, Male; F, Female; N, No; LLW, Lower limb weakness. |
|||||||
Table III: Comparison of IONM findings of the current study with the study conducted by Yoshida et al.
|
Variable |
Yoshida et al.17 |
Current Study |
|
|
Spinal deformity cases (n) |
1009 |
170 |
|
|
Surgical Technique |
Posterior corrective fusion |
Posterior corrective fusion |
|
|
Anesthesia |
TIVA (Propofol + Fantanyl) |
TIVA (Propofol + Dexmedetomidine) |
|
|
TcMEP Alarm criteria |
≥70% amplitude reduction |
≥70% amplitude reduction |
|
|
IONM findings |
Signal alert, n (%) |
57 (5.65) |
27 (15.9) |
|
Rescued cases, n (%) |
35 (61.4) |
16 (59.26) |
|
|
No improvement, n (%) |
22 (38.6) |
11 (40.74) |
|
|
Timing of signal alert |
Rod de-rotation (%) |
36.8 |
25 |
|
Tapping for screws (%) |
- |
16 |
|
|
Pedicle screw insertion (%) |
12.3 |
25 |
|
|
Compression/distraction (%) |
7 |
15 |
|
|
Decompression (%) |
5.3 |
12 |
|
|
Others / surgery unrelated (%) |
10.5 |
- |
|
|
Postoperative neurological deficit (%) |
2.2 |
5.29 |
|
|
Efficacy of TcMEP |
Sensitivity (%) |
93.3 |
100 |
|
Specificity (%) |
91 |
98.6 |
|
|
PPV (%) |
35 |
81.81 |
|
|
NPV (%) |
99.6 |
100 |
|
Out of 11 cases of sustained signal drop, 9 (5.29%) cases had exhibited post-operative neurological deficit (true positive), whereas 2 (1.17%) cases did not show any postoperative neurological deficit (false positive). Sustained signal drop was significantly higher (p=0.039) in the cases of congenital scoliosis as compared to idiopathic scoliosis. Among 27 cases of evoked potential changes, 12 (7.1%) cases shown isolated TcMEP changes whereas 15 (8.8%) cases had both TcMEP and SSEP changes. None of the cases has shown isolated SSEP changes. Total of 3 (1.76%) cases have exhibited high frequency neurotonic s-EMG activities in various isolated muscles during corrective measures. Details of evoked potential changes and neurological deficits in all cases are presented in Table II. The efficacy of multimodal IONM in current study calculated using two by two contingency revealed sensitivity of 100%, specificity of 98.6%, positive predictive value of 81.81% and negative predictive value of 100%.
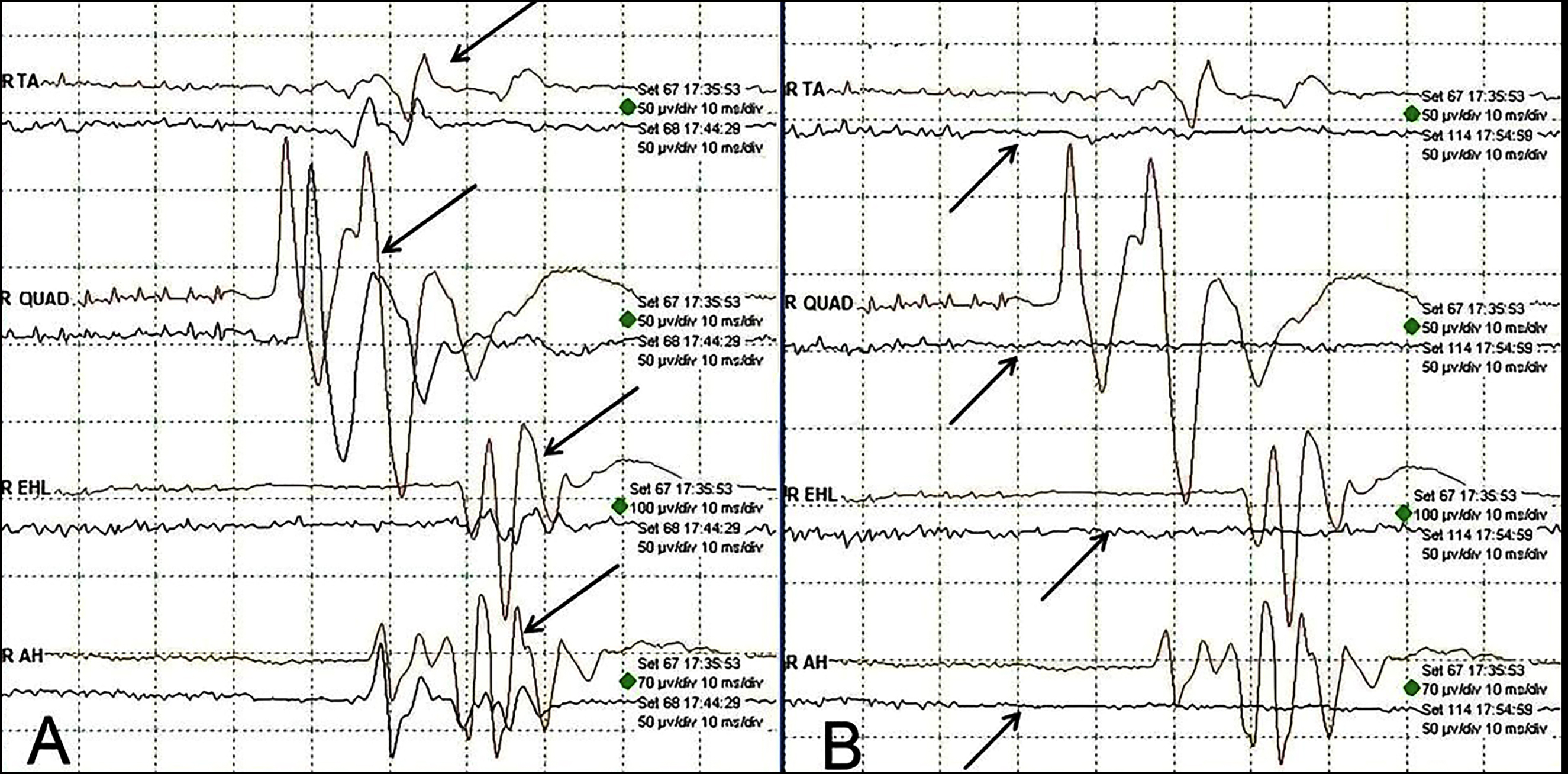 Figure 1: Intraoperative neurophysiological monitoring showing transcranial motor evoked potential data of 18-year patient of congenital scoliosis mentioned at serial 27 of Table II. (A) Baseline data of TcMEPs (arrows) in the right lower limb. (B) Complete loss of TcMEPs (arrows) during the surgery while de-rotating rod.
Figure 1: Intraoperative neurophysiological monitoring showing transcranial motor evoked potential data of 18-year patient of congenital scoliosis mentioned at serial 27 of Table II. (A) Baseline data of TcMEPs (arrows) in the right lower limb. (B) Complete loss of TcMEPs (arrows) during the surgery while de-rotating rod.
DISCUSSION
Corrective surgery of scoliosis without postoperative neurological deficit always remained challenge to the surgical team.11 Previously, to assess the gross motor functions of the patient during the surgery, surgeons were used to perform the Stagnara wake-up test in which intraoperative anaesthesia is reduced and patient is asked to move his/her limbs.12 Application of IONM in complex spinal surgeries has become an essential adjunct but its efficacy is still debatable since it has never been tested in clinical trials.13 Nevertheless, multimodal IONM has been reported to have the highest reliability in spine deformity surgeries in reducing postoperative neurological deficit.14 Findings of the current study are comparable to similar studies reporting IONM efficacy in spinal surgeries.15 In the present study, signal change was observed in significant number of cases which identified these cases as being at risk of postoperative neurologic deficit. Immediate warnings were issued to the surgical team which led to the exercising prompt corrective measures (mechanical adjustments). At the same time administration of dexamethasone and maintenance of adequate blood pressure was also ensured by close cooperation of anaesthesiologists. These corrective measures reversed adverse surgical events in significant number of cases explaining the crucial role of IONM in enhancing the safety and success of surgical procedures involving the nervous tissue.
In the present study, 5.29% cases have developed new postoperative neurological deficit that is notably higher than the study conducted by Nassef et al. where they reported 3.3% incidence of new neurologic deficit in cases of idiopathic scoliosis operated under multimodal IONM.16 The difference may be explained by almost half of the cases of congenital scoliosis in the sample of the current study which carries a greater risk of complications. Moreover the post-operative neurologic deficit in isolated cases of idiopathic scoliosis in the current study was only 1.2% (Table I). In another prospective multicenter study, Yoshida et al. evaluated the role of IONM in monitoring of various high-risks spinal surgeries.17 Comparison of IONM findings in spinal deformity cases of their study with the current study is presented in Table III. Stephen et al. retrospectively studied IONM Changes in paediatric coronal spinal deformity surgery in 97 cases out of which they observed signal change of MEPs and SSEPs in 27 (27.8%) cases. By exercising corrective measures and careful perioperative monitoring and management signal recovery to some degree occurred in all patients (100% recovery in 20 cases while reaming cases have shown recovery between 25 – 75%). None of the patients in their study had displayed postoperative neurological deficit which signifies the importance of IONM.18
In another study, the data of IONM on 37 paediatric 3-column osteotomies was presented. The authors observed signal alerts in 21 (57%) cases. Corrective measures restored complete recovery of signals in 16 (43.5%) patients whereas 5 (13.5%) displayed partial recovery of signals with associated postoperative neurological deficits however all regained full function within 3 months of surgery.19 Another study evaluated the efficacy of multimodality IONM in severe thoracic deformity corrected by posterior vertebral column resection. The study observed monitoring events in 27 (32.9%) cases. Only 5.1% of these events were reported to be caused by hypotension while remaining all the signal change events were due to surgical maneuvers. Prompt surgical interventions caused complete recovery of signals in 18 (21.9%) cases whereas 9 (11%) cases in which monitoring data did not return to the baseline had postoperative neurologic deficit.20
Although multimodality IONM has expanded surgical scope by enabling the expansion of surgical procedures for more complex surgeries that might otherwise carry higher risks of neurological deficit, the procedure carries certain limitations. The most important limitation is false positives and false negatives results. The monitoring signals may not always accurately reflect the actual neurological status, leading to potential misinterpretations. Other limitations include skill-dependent interpretation of data because variability in expertise may impact the reliability of the monitoring results and cost-effect as financial benefits in terms of reduced complications are not always clear. Balancing the benefits against the costs is an ongoing debate.21
The current study has certain limitations, one of them is in terms of TIVA protocol. The drug ramifentanil (commonly used agent) was not used in TIVA due to budget constraints and unavailability. Instead, dexmedetomidine was used in all cases along with propofol which has suppressive effect on evoked potentials. However, the depth of anaesthesia was strictly monitored through BIS to avoid suppressive effect of anaesthesia on evoked potentials. Cost effect and unaffordability of the patients also limited the selection of modalities to monitor as per existing standard practice.
CONCLUSION
Multimodal IONM reduces the incidence of postoperative neurological deficit in corrective surgery of scoliosis by effectively detecting neurologic injury during the course of surgery. Monitoring events alert surgical team to exercise immediate corrective measures which likely results in the recovery of lost signals and predict the favourable outcome in high-risk spinal surgeries.
ETHICAL APPROVAL:
Formal permission was obtained from the Ethical Review Committee of the Combined Military Hospital, Rawalpindi (IRB Serial No. 391 dated 2 December 2022) prior to initiation of the research.
PATIENTS’ CONSENT:
As it was a retrospective study, informed verbal consent was obtained from the patients on phone calls prior to the study.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
MT, MAQ: Study concept.
SA: Designing of study and data analysis.
SA, WA, BS, SI: Drafting of the manuscript.
SA, BS, SI: Acquisition of data:
MT, MAQ, WA, Interpretation of data.
MT, MAQ, WA, BS, SI: Critical revision of the manuscript.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Chen J, Yang JF, Deng YL, Shao XX, Huang ZF, Yang JL, et al. A retrospective study of surgical correction for spinal deformity with and without osteotomy to compare outcome using intraoperative neurophysiological monitoring with evoked potentials Med Sci Monit 2020; 26:e925371. doi: 10. 12659/MSM.925371.
- Rajappa D, Khan MM, Masapu D, Manchala R, Rudrappa S, Gopal S, et al. Multimodal intraoperative neurophysiological monitoring in spine surgeries: The experience at a spine centre through years. Asian Spine J 2021; 15(6):728-38. doi: 10.31616/asj.2020.0400.
- Wi SM, Lee HJ, Kang T, Chang SY, Kim SM, Chang BS, et al. Clinical significance of improved intraoperative neuro-physiological monitoring signal during spine surgery: A retrospective study of a single-institution prospective cohort. Asian Spine J 2020; 14(1):79-87. doi: 10.31616/ asj.2019.0025.
- George J, Das S, Egger AC, Chambers RC, Kuivila TE, Goodwin rc. influence of intraoperative neuromonitoring on the outcomes of surgeries for pediatric scoliosis in the united states. Spine Deform 2019; 7(1):27-32. doi: 10. 1016/j.jspd.2018.05.013.
- Kim JE, Kim JS, Yang S, Choi J, Hyun SJ, Kim KJ, et al. Neurophysiological monitoring during anterior cervical discectomy and fusion for ossification of the posterior longitudinal ligament. Clin Neurophysiol Pract 2021; 6:56-62. doi: 10. 1016/j.cnp.2021.01.001.
- Ali L, Jahangiri FR, Ali A, Belkhair S, Elalamy O, Adeli G, et al. Emerging super-specialty of neurology: Intraoperative neurophysiological monitoring (ionm) and experience in various neurosurgeries at a tertiary care hospital in Doha, Qatar. Cureus 2021; 13(12):e20432. doi: 10.7759/cureus. 20432.
- Kim K. Intraoperative neurophysiology monitoring for spinal dysraphism. J Korean Neurosurg Soc 2021; 64(2):143-50. doi: 10.3340/jkns.2020.0124.
- Charalampidis A, Jiang F, Wilson JRF, Badhiwala JH, Brodke DS, Fehlings MG. The use of intraoperative neurophysio-logical monitoring in spine surgery. Global Spine J 2020; 10(1 Suppl):104S-14S. doi: 10.1177/2192568219859314.
- Falowski SM, Benison A. Prospective analysis utilizing intraoperative neuromonitoring for the evaluation of inter-burst frequencies. J Pain Res 2021; 14:703-10. doi: 10. 2147/JPR. S298797.
- Quraishi NA, Lewis SJ, Kelleher MO, Sarjeant R, Rampersaud YR, Fehlings MG. Intraoperative multimodality monitoring in adult spinal deformity: analysis of a prospective series of one hundred two cases with independent evaluation. Spine (Phila Pa 1976) 2009; 34(14):1504-12. doi: 10.1097/BRS. 0b013e 3181a87b66.
- Biscevic M, Sehic A, Krupic F. Intraoperative neuro-monitoring in spine deformity surgery: modalities, advantages, limitations, medicolegal issues - surgeons' views. EFORT Open Rev 2020; 5(1):9-16. doi: 10.1302/ 2058-5241.5. 180032.
- Strike SA, Hassanzadeh H, Jain A, Kebaish KM, Njoku DB, Becker D, et al. Intraoperative neuromonitoring in pediatric and adult spine deformity surgery. Clin Spine Surg 2017; 30(9):E1174-E81. doi: 10.1097/BSD. 0000000000000388.
- Goonasekera C, Jones H, Lawrence R, Hanrahan J, Iyer P, Nijhawan A. exploring the utility of neuro-monitoring in neurosurgery: The users' perspective in a single center. Saudi J Anaesth 2021; 15(1):7-13. doi: 10.4103/sja.SJA_ 862_20.
- Lall RR, Lall RR, Hauptman JS, Munoz C, Cybulski GR, Koski T, et al. Intraoperative neurophysiological monitoring in spine surgery: indications, efficacy, and role of the pre-operative checklist. Neurosurg Focus 2012; 33(5):E10. doi: 10.3171/ 2012.9.FOCUS12235.
- Wi SM, Park SM, Chang SY, Lee J, Kim SM, Chang BS, et al. Surgical Causes of Significant Intraoperative Neuro-monitoring Signal Changes in Three-Column Spinal Surgery. Asian Spine J 2021; 15(6):831-9. doi: 10.31616/asj.2021. 0078.
- Nassef M, Splinter W, Lidster N, Al-Kalbani A, Nashed A, Ilton S, et al. Intraoperative neurophysiologic monitoring in idiopathic scoliosis surgery: a retrospective observational study of new neurologic deficits. Can J Anaesth 2021; 68(4): 477-84. doi: 10.1007/s12630-020-01898-9.
- Yoshida G, Ando M, Imagama S, Kawabata S, Yamada K, Kanchiku T, et al. Alert timing and corresponding intervention with intraoperative spinal cord monitoring for high-risk spinal surgery. Spine (Phila Pa 1976) 2019; 44(8):E470-E9. doi: 10.1097/BRS.0000000000002900.
- Lewis SJ, Wong IHY, Strantzas S, Holmes LM, Vreugdenhil I, Bensky H, et al. Responding to intraoperative neuro-monitoring changes during pediatric coronal spinal deformity surgery. Global Spine J 2019; 9(1 Suppl): 15S-21S. doi: 10.1177/2192568219836993.
- Jarvis JG, Strantzas S, Lipkus M, Holmes LM, Dear T, Magana S, et al. Responding to neuromonitoring changes in 3-column posterior spinal osteotomies for rigid pediatric spinal deformities. Spine (Phila Pa 1976) 2013; 38(8): E493-503. doi: 10.1097/BRS.0b013e3182880378.
- Huang ZF, Chen L, Yang JF, Deng YL, Sui WY, Yang JL. Multimodality intraoperative neuromonitoring in severe thoracic deformity posterior vertebral column resection correction. World Neurosurg 2019; 127:e416-e26. doi: 10.1016/j. wneu.2019.03.140.
- Tamkus AA, Rice KS, McCaffrey MT. Perils of intraoperative neurophysiological monitoring: analysis of "false-negative" results in spine surgeries. Spine J 2018; 18(2):276-84. doi: 10.1016/j.spinee.2017.07.005.